How to select materials in a chloride-corrosion environment
How to select materials in a chloride-corrosion environment
1. Stress Corrosion Cracking (SCC)
Stress corrosion occurs when stainless steel is exposed to a corrosive medium containing oxygen and chloride ions. Stress corrosion failures account for approximately 45% of all corrosion-related failures.
Common Prevention Measures:
Material Selection: Use materials resistant to stress corrosion, such as high-purity austenitic chromium-nickel steel, high-silicon austenitic chromium-nickel steel, high-chromium ferritic steel, and ferritic-austenitic duplex steel (which offers the best stress corrosion resistance).
-Stress Control: Minimize stress concentration during assembly, ensure minimum residual stress in areas exposed to the medium, avoid impact damage or scratches, and strictly follow welding procedures.
Strict Operation Protocols: Control raw material composition, flow rate, medium temperature, pressure, and pH value. Add corrosion inhibitors within permissible process limits. For chromium-nickel stainless steel used in oxygen-dissolved chloride media, reduce oxygen content to below 1.0 × 10⁻⁶. For instance, adding 150.0 × 10⁻⁶ nitrate and 0.5 × 10⁻⁶ sodium sulfite to water containing 500.0 × 10⁻⁶ chloride ions can significantly improve performance.
2. Pitting Corrosion and Prevention
Pitting corrosion typically occurs in stagnant media. Pits usually grow along the direction of gravity or laterally and accelerate once formed. In chloride-containing aqueous solutions, the protective oxide film on stainless steel dissolves, forming small pits (20–30 μm in diameter) on the base metal, which act as pitting nuclei. Chloride ions can promote the growth of these nuclei into larger pits.
Common Prevention Measures:
- Add elements like molybdenum (Mo), nitrogen (N), and silicon (Si) to stainless steel, and increase chromium (Cr) content.
- Reduce the concentration of chloride ions in the medium.
- Use corrosion inhibitors to stabilize the passive film and facilitate repassivation of damaged films.
- Apply external cathodic protection to suppress pitting.
3. Crevice Corrosion
Crevice corrosion has a similar mechanism to pitting corrosion and occurs due to localized concentration of chloride ions in occluded areas. This type of corrosion is common in flange gaskets, lap joints, bolt-nut crevices, and the gaps between heat exchanger tubes and tube sheets. It is closely related to the concentration of stagnant solutions in crevices. Once crevice corrosion develops, it significantly increases the likelihood of inducing stress corrosion cracking.
Common Prevention Measures:
- Minimize the number of crevices in design and assembly.
- Use materials with high resistance to crevice corrosion (e.g., duplex stainless steel, or stainless steel with added molybdenum).
- Seal crevices to prevent the entry of chloride-containing solutions.
- Regularly clean and maintain to remove any stagnant solution in crevices.
4. Pitting Corrosion
All metal materials contain non-metallic inclusions to some degree. In stainless steel, non-metallic compounds may form pitting corrosion under the action of chloride ions. Chloride ions migrate into pits, while positively charged metal ions move outward, creating a concentration gradient that accelerates the pitting process. Adding molybdenum (Mo) significantly improves resistance to pitting corrosion; the higher the Mo content, the better the performance.
Prevention Measures:
- Select Mo-containing stainless steels for better pitting resistance.
- Ensure a smooth surface finish to reduce sites for pitting initiation.
- Use inhibitors or external protection methods to stabilize the passive film.
By combining appropriate material selection, design optimization, and strict operational controls, the risk of chloride-induced corrosion can be effectively minimized.
II. Conditions of Use for Various Stainless Steels in Chloride-Containing Aqueous Solutions
1. 304 Stainless Steel
- The most affordable and widely used austenitic stainless steel, suitable for general organic and inorganic media. Common applications include food, chemical, and nuclear industries.
- Examples of suitable conditions:
- Nitric acid: Concentration <30%, temperature ≤100°C, or concentration ≥30%, temperature <50°C.
- Carbonates, ammonia water, alcohols at temperatures ≤100°C.
- Poor resistance to sulfuric and hydrochloric acid. Particularly sensitive to crevice corrosion caused by chloride-containing media (e.g., cooling water).
2. 304L Stainless Steel
- Similar corrosion resistance and applications as 304, but with lower carbon content (≤0.03%), offering better resistance to intergranular corrosion (including in weld zones) and improved weldability. Suitable for partially or fully welded plate heat exchangers (PHE).
3. 316 Stainless Steel
- Resistant to general organic and inorganic media such as natural cooling water, cooling tower water, soft water, carbonates, acetic acid (<50%), and dilute alkalis.
- Superior to 304 in resistance to chlorides due to ~2% molybdenum (Mo) content, making it suitable for seawater and chloride-containing environments, effectively replacing 304.
4. 316L Stainless Steel
- Similar to 316 but with lower carbon content (≤0.03%), providing better weldability and corrosion resistance in welded zones. Ideal for PHE applications.
5. 317 Stainless Steel
- Offers longer service life than 316 due to slightly higher Cr, Mo, and Ni content, improving resistance to crevice corrosion, pitting, and stress corrosion cracking.
6. AISI 904L or SUS 890L Stainless Steel
- A cost-effective austenitic stainless steel with superior corrosion resistance, especially suitable for sulfuric acid, phosphoric acid, and halide environments (e.g., Cl⁻ and F⁻).
- High Cr, Ni, and Mo content provides excellent resistance to stress corrosion, pitting, and crevice corrosion.
7. Avesta 254 SMO
- A super low-carbon stainless steel improved from 316 by increasing Mo content.
- Offers excellent resistance to chloride-induced pitting and crevice corrosion, suitable for saline water and inorganic acids where 316 is insufficient.
8. Avesta 654 SMO
- Contains higher levels of Cr, Ni, Mo, and N than 254 SMO, offering superior resistance to chloride corrosion. Suitable for cold seawater applications.
9. RS-2 (OCr20Ni26Mo3Cu3Si2Nb) Stainless Steel
- A domestic Cr-Ni-Mo-Cu stainless steel with pitting and crevice corrosion resistance comparable to 316 but better stress corrosion resistance. Applicable to concentrated sulfuric acid (90–98%, ≤80°C) with a corrosion rate ≤0.04 mm/year.
10. Incoloy 825
- A high-end Ni (40%)–Cr (22%)–Mo (3%) stainless steel.
- Resistant to all concentrations of sulfuric acid at low temperatures and caustic alkalis (e.g., 50–70% NaOH).
- Sensitive to chloride-induced crevice corrosion and less suitable for plate applications due to poor stamping properties.
11. Alloy 31
- An improved version of 904L with higher Mo and N content (standard 6% Mo stainless steel).
- Superior corrosion resistance compared to 904L, particularly in sulfuric acid (20–80% concentration, 60–100°C) where its performance surpasses C-276.
12. Alloy 33
- A fully austenitic chromium-based stainless steel with corrosion resistance comparable to Ni-Cr-Mo alloys like Inconel 625.
- Performs well in acidic and alkaline media (e.g., nitric acid, HF-nitric acid mixtures).
- Excellent in concentrated nitric acid (96–99%, ≤150°C), seawater, and boiling phosphoric acid solutions (≤85%, ≤150°C).
13. C-2000 Alloy
- A Ni-based alloy developed in the 1990s, offering exceptional corrosion resistance in dilute sulfuric acid, hydrochloric acid, boiling phosphoric acid (≤50% concentration), and hot chloride media.
- Outperforms C-276 and C-22 in many environments but less effective in concentrated sulfuric acid (≥70%).
14. 59 Alloy
- A Ni-based alloy with slightly higher Ni content (59%) and no Cu or W.
- Offers the best corrosion resistance, thermal stability, formability, and weldability among Ni-based alloys. Widely used in sulfuric acid, hydrochloric acid, hydrofluoric acid, and chloride-oxygen-low pH environments since commercialization in 1990.
These stainless steels offer varied levels of resistance to chloride-induced corrosion, allowing for material selection based on specific environmental and operational conditions.
Besides above mentioned material inconel 625 globe valve this special material also has it’s widely applied.
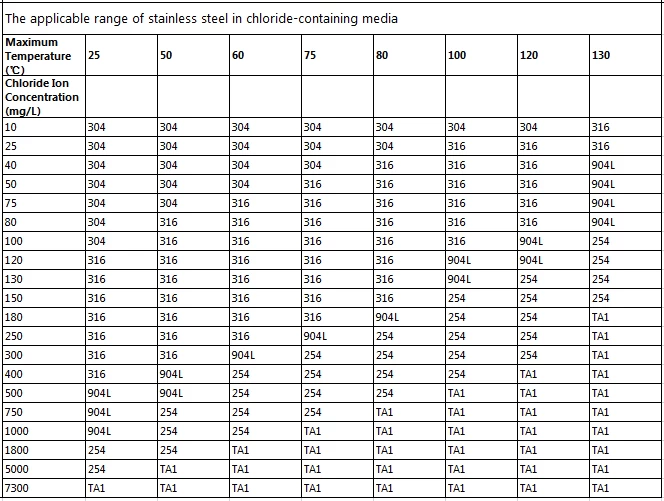
III. Material Selection Table Based on Temperature and Chloride Ion Concentration
 +86 512 68781993
+86 512 68781993